

Involvement of Nrf2 and Keap1 in the Induction of Defense Enzymes/Proteins in Response to a Hazardous Environment
Å@Molecular evolution has bestowed upon animals a number of defense systems enabling them to survive in a hazardous environment. The response mechanism to electrophilic intermediates of dietary chemicals and reactive oxygen species (ROS) represents one of the most important cellular defense mechanisms against environmental toxins (Figure 1). This electrophile-response mechanism has been observed in the rat, mouse and human, in the form of a group of detoxification enzymes and antioxidant proteins. Transcription of these defense enzymes/proteins is coordinately induced through the electrophile responsive element/ antioxidant responsive element (EpRE/ARE).

Fig. 1
Å@Transcription factor Nrf2 (NF-E2-related factor 2) and ECH (Erythroid-derived CNC Homology protein) have been suggested to act as indispensable regulators of the cellular response. Nrf2 and ECH belong to the CNC family of transcription factors, whose members share a highly conserved basic region-leucine zipper (bZip) structure. Nrf2 forms a heterodimer with a small Maf protein and binds to its cognate DNA sequence, NF-E2-binding sequence or ARE (Figure 2). Using the nrf2 gene knockout mouse, we recently demonstrated that Nrf2 deficiency largely abolishes the constitutive and/or inducible expression of defense enzyme genes in response to oxidative and xenobiotic stress. We also demonstrated that Nrf2 plays a protective role against acetaminophen hepatotoxicity by regulating drug metabolizing and antioxidant enzyme genes through the ARE. Loss of Nrf2 expression significantly enhanced the susceptibility of mice to benzo[a]pyrene carcinogenesis and completely abolished the chemoprotective properties of phase 2 inducers such as oltipraz. Based on these observations, we conclude that Nrf2 is the principal regulator of ARE-mediated transactivation.
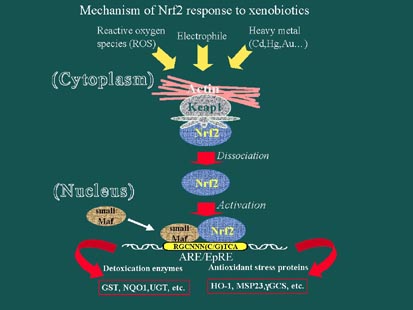
Fig. 2
Å@A sequence similarity alignment of human Nrf2 and chicken ECH proteins identified six highly conserved regions, referred to as Neh1 to Neh6 (Nrf2-ECH homology). Of these regions, Neh1 corresponds to the bZip domain, whereas Neh2 is the domain which negatively regulates Nrf2 function through its interaction with Keap1 (Kelch-like ECH associating protein 1), an actin cytoskeleton binding protein homologous to the Drosophila protein Kelch. When overexpressed in transfected cells, Nrf2 directly localizes in nuclei and acts as a potent transactivator. In contrast, the concomitant expression of Keap1 sequesters Nrf2 from the nuclei, hence repressing Nrf2 activity. Importantly, using a primary culture of peritoneal macrophages, we found that electrophilic agents, such as diethylmaleate (DEM), result in the nuclear accumulation of Nrf2 and activation of reporter gene transcription.
REFERENCE