(Published Nov 11, 2020)
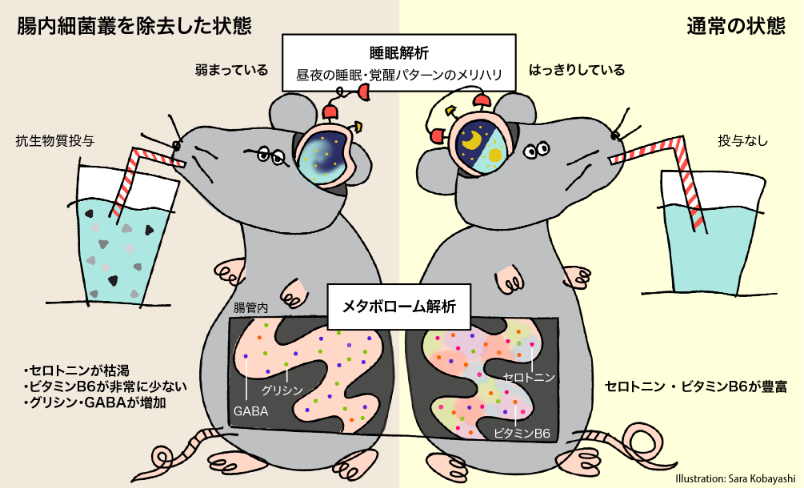
The intestinal environment, including the gut flora, has been shown to interact with brain function.
In this study, the relationship between the intestinal flora and sleep was investigated using mice in which the intestinal flora was removed by chronic antibiotic administration. A metabolome analysis of the contents of the cecum determined that the state of intestinal metabolism in “intestinal flora-removed” mice showed significant changes in the metabolic pathways of amino acids involved in neurotransmitter synthesis compared to normal mice. In particular, vitamin B6 was significantly reduced and serotonin, which regulates nerve function, was depleted.
On the other hand, a significant increase in glycine and gamma-aminobutyric acid (GABA), which suppress the activity of nerve cells, was observed.
When sleep was analyzed using EEG and EMG, “intestinal flora-removed” mice had decreased sleep in the light period (sleep period) and increased sleep in the dark period (active period). The day and night, sleep / wakefulness pattern was weakened. It was also found that theta waves, which are the characteristic brain waves components of REM sleep with cerebral cortex activity, were reduced. These findings suggest that removal of the gut flora may reduce sleep quality.
It is expected that further research may allow new methodologies for promoting improved health through a deeper understanding of the interaction between the intestinal environment and brain function (gut-brain axis) and eating habits.
Limited rejuvenation of aged hematopoietic stem cells in young bone marrow niche
Key Point
→
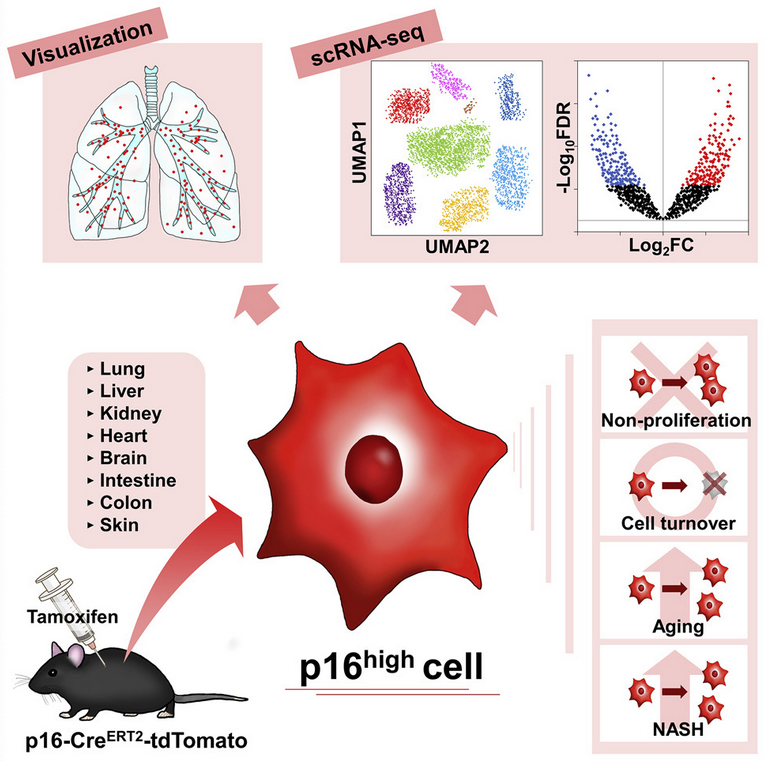
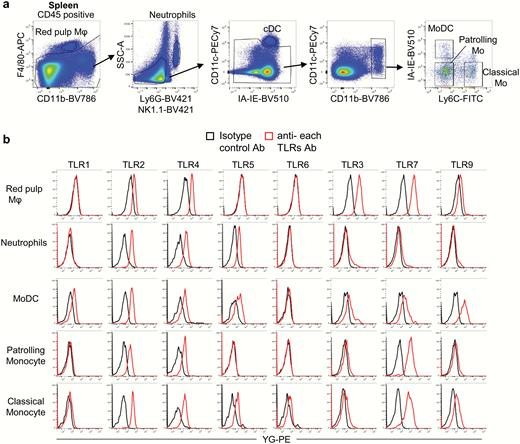
Generation of a p16ʰⁱᵍʰ Reporter Mouse and Its Use to Characterize and Target p16ʰⁱᵍʰ Cells In Vivo
(Available online Sept 18th, 2020)
Key Points
• p16high cells are detectable in all tissues, are enriched with age, and do not proliferate.
• p16high cells exhibit heterogenous senescence-associated phenotypes.
• Elimination of p16high cells ameliorates steatosis and inflammation in a NASH model.
→
Nrf2 contributes to the weight gain of mice during space travel
Following experiments on the International Space Station (ISS) it has been reported that deletion of Nrf2, a master regulator of stress defense pathways, affects the health of mice flown for 31 days in Earth’s orbit. All flight mice returned safely to Earth where transcriptome and metabolome analysis revealed that the stresses of space travel evoked ageing-like changes of plasma metabolites, and activated the Nrf2 signaling pathway. Especially, Nrf2 was found to be important for maintaining homeostasis of white adipose tissues.
The study opens approaches for future space research utilizing murine gene knockout-disease models, and provides insights to mitigating space-induced stresses that currently limit potential for space exploration by humans.


→ Nature - Communications Biology (2020)
DOI: 10.1038/s42003-020-01227-2
Professor Shinji Fukuda Featured in Financial Times 2020 List of Asia-Pacific High-Growth Companies
(from April 2020)
Transborder Medical Research Center (TMRC) member Professor Shinji Fukuda of Keio University and CEO of Megabologenomic Inc., has been featured in a Financial Times article recognising his company’s research and success, while ranking 50th on the FT list of “Asia-Pacific High-Growth Companies 2020”.
This ranking is based on a survey targeting companies whose headquarters are located in the Asia-Pacific region and lists the top 500 companies with consideration to the compound annual growth rate of their revenues across 2015 to 2018.
The company is built upon research in the field of Metabologenomics in collaboration with TMRC. The Metabologenomic Inc technology and service is now providing important positive economic and community health benefits within the Asia-Pacific region.
The full article is available on the Financial Times website.
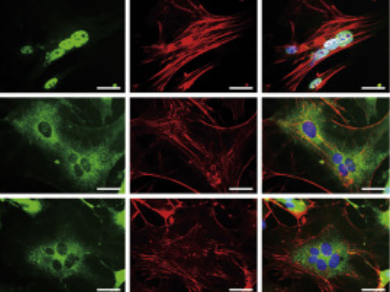
Highlights
• ECM comparable with native myocardium promotes cardiac reprogramming.
• Soft ECM promotes cardiac reprogramming via YAP/TAZ/fibroblast signaling inhibition.
• Soft ECM promotes Sendai virus vector-mediated cardiac reprogramming.
→
Estrogen Receptor β Controls Muscle Growth and Regeneration in Young Female Mice
Key Points
• ERβ is essential for muscle regeneration in female mice.
• Inactivation of ERβ causes an increase in apoptosis.
• ERβ is required for satellite cell population expansion
→
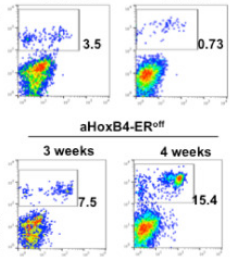
Key Points
• HoxB4 activity is required for generating long-term bone marrow engraftment from iPSC-derived HSCs.
• The intranuclear localization of HoxB4 protein in HSC-precursors controls differentiation arrest through enhanced expression of genes governing hematopoietic development.