
 |
Regulatory Mechanisms of GATA-1 Gene Expression
|
|
|
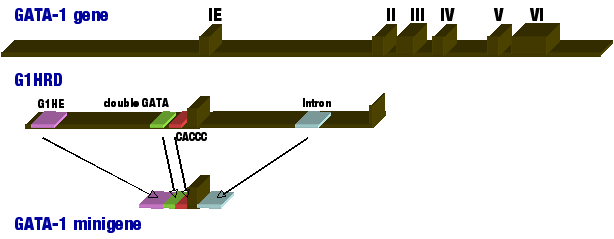
@The GATA family of transcription factors is a group of DNA binding proteins whose members bind to a consensus sequence of 5'-(A/T)GATA(A/G)-3' through highly conserved zinc finger DNA-binding domains. GATA-4,5, and 6 are expressed in various mesoderm- and endoderm-derived tissues, such as heart, liver, and gut, where they regulate tissue-specific gene expression. On the other hand, GATA-1, 2, and 3 are expressed in specific lineages of hematopoietic cells where they regulate distinct steps during the development of the hematopoietic system including erythroid cells, eosinophils, megakaryocytes and T-lymphocytes. GATA-1 has specifically been shown to play critical roles in cell maturation and differentiation within erythroid-lineages by regulating expression of genes such as Erythroid 5-aminolevulinate synthase (ALAS-E; heme synthase) and alpha/ΐ-globin genes. Thus, understanding the regulatory mechanisms of GATA1 gene itself holds the key to understanding erythroid differentiation. @The mouse Gata1 gene consists of two alternative first exons (IT - testis specific; IE- erythroid specific) and five translated exons. In order to understand the Gata1 regulatory mechanism, we have constructed transgenic mouse models containing LacZ, luciferase, or GFP reporter genes located immediately downstream of Gata1 promoter. Using these mouse models, we have shown that 3.9kb region upstream of IE exon and 4.2kb region of the first intron can sufficiently recapitulate Gata1 gene expression in primitive and definitive erythroid cells and magakaryocytes (IE3.9intLacZ). Thus, we refer to the IE3.9int fragment as the GATA-1 hematopoietic regulatory domain (G1HRD; Fig 1). Further investigation of G1HRD has revealed that G1HE region, double GATA-, and CACCC-boxes are all necessary for Gata1 expression in primitive erythroid cells, whereas the addition of the first intron region drives Gata1 expression in definitive erythroid cells. The four regions were placed in tandem to create the "GATA-1 minigene," which was sufficient to duplicate endogenous Gata1 gene expression in transgenic mouse models (Fig 1). Interestingly, many of Gata1 regulatory regions contain GATA-boxes, suggesting Gata1 autoregulation by GATA-family of transcription factors. Indeed, we have shown that Gata-1 homodimer-mediated autoregulation of Gata1 gene expression in transgenic zebrafish model. |
||
 |
||
|
Figure 1. Schematic drawing of mouse Gata1 gene organization. GATA-1 minigene is comprised of G1HE, double GATA box, CACCC box, and the first intron region placed in tandem.
|
||
|
|
||
|
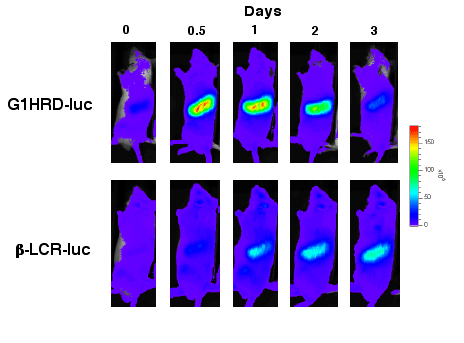
@In order to bypass the traditionl method of bone marrow biopsy, we successfully generated transgenic mouse models expressing G1HRD- or ΐ-globin promoter (ΐ-LCR) dependent luciferase, which allowed us to follow real-time changes in respective genes' expression by in vivo bioluminescence imaging (IVIS imaging system; Xenogen, Alameda, CA). The introduction of anemia or exposure to hypoxic conditions of these animals revealed that the process of stress erythropoiesis is marked by the intial increase in plams erythropoietin level, which tirggers the increase in the epxression of GATA-1, followed by the increase in ΐ-globin gene expression. Our aim is to decipher the pattern of gene expression critical of hemopoiesis to understand the underlying mechanism of hematopoietic homeostasis. |
||
 |
||
| Figure 2DTransgenic mice expressing G1HRD- dependent luciferase or ΐ-LCR-dependent luciferase. Under normal condition, luciferase acitivty cannot be detected in either mice. However, during the recovery phase from anemia, Gata1 shows increased expression (top panel) followed by the expression of ΐ-globin (bottom panel). |