Integrated Research Division
Regenerative Medicine
Keywords:
Muscle Stem Cells, Skeletal Muscle Regeneration, Muscular Dystrophy, Direct Reprogramming
Development of a new approach for the treatment of muscle diseases, including muscular dystrophy, cachexia and sarcopenia, by understanding the molecular mechanisms of the muscle stem cells.
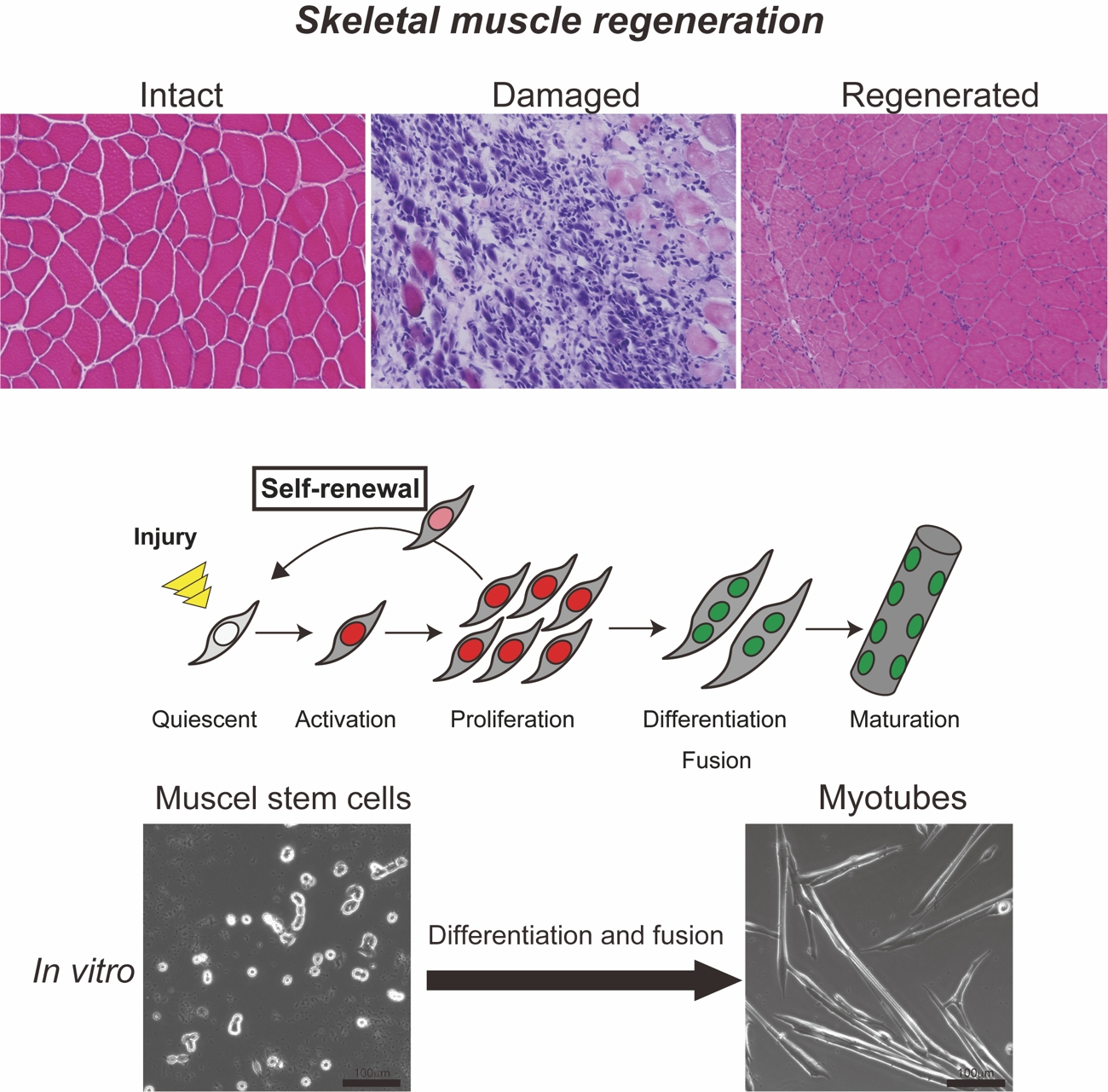
Skeletal muscles have an impressive capability to regenerate after severe injury. This regeneration capacity and the life-long maintenance of skeletal muscle tissues are mediated by muscle stem cells (MuSCs), also known as ‘satellite cells’ for their position underneath the basal lamina of muscle fibers. Muscle stem cells are normally resting, but become activated to proliferate and differentiate into multinucleated myofibers in response to muscle injury or intense exercise. Despite their amazing ability to regenerate, the number and the function of muscle stem cells declines with many disease and disorders, including aging and a family of muscular dystrophies, which leads to failure of regeneration. Therefore, we are working to understand the molecular mechanisms underlying skeletal muscle regeneration, and to develop the strategy to prevent the loss of “stemness” in the muscle stem cells. The results of our research will be key for regenerative medicine in treating many muscle diseases such as Duchenne muscular dystrophy (DMD) and sarcopenia. Research
(1) Molecular regulation of muscle stem cell function.
It is well known that adult stem cell populations, including muscle stem cells, are heterogenous, but we still don’t know why. This current project is deciphering the role of each MuSCs populations during muscle differentiation and regeneration.
(2) The development of stem cell-based therapies for muscle diseases by direct reprogramming.
The capacity of self-renewal to make copies of muscle stem cells declines with aging and muscle diseases. Once the muscle stem cells are lost, it is almost impossible to replenish the muscle stem cells population within our body. We are addressing to make muscle stem cells/progenitors by identifying new targetable pathways.
(3) Improvement of direct cardiac reprogramming.
Similar to skeletal muscle, cardiac muscle is also striated and organized into sarcomeres. However, there are no equivalent cells to the satellite cells found in skeletal muscle. Thus, the heart is unable to regenerate cardiac muscle after a myocardial infarction, and the cardiac muscle is eventually replaced by scar tissue. For this problem, Dr. Masaki Ieda (Department of Cardiology) demonstrated that in situ generation of new cardiomyocytes from endogenous cardiac fibroblasts by direct reprogramming (Ieda M et al. Cell. 2010). We are further characterizing different pathways that enhance the cardiac direct reprogramming efficiency.
Research Division Leader
Assistant Professor Ryo Fujita

Research Division Leader
See Associated External Research Website